Understanding the Gut Microbiome Through the Lens of the OMX Organic Metabolomics Test

Introduction – Metabolites from the Gut Microbiome
We can't talk about the human metabolome without talking about metabolites from the gut microbiome. In fact, over 10% of the human metabolome is directly associated with gut microbial metabolism.1 The Diagnostic Solutions GI-MAP™ stool test makes it possible to directly identify and measure the quantities of bacteria and fungi in the stool. But the OMX™ Organic Metabolomics test allows you to look at the gut bacteria from a different angle — from their byproducts or metabolites. When taken together, these two tests give you a more complete picture of a person's gut microbiome.
The gut microbiome has been described as an organ — and a metabolic organ at that. Indeed, 95% of the dietary polyphenols that you eat pass along to the colon where they are fermented by gut microorganisms.2
Bacterial metabolites can harm the host3 or they can enhance biological activity and promote health of the host.4 The best example of bacterial metabolism improving the bioactivity of a polyphenol is equol, which benefits hormone balance, cardiovascular health, and cancer risk.
There is evidence that equol is more bioactive than its parent isoflavone [daidzein] in a range of areas including estrogenic and anti-estrogenic activity, antioxidant capacity and potential anti-cancer effects [99]. Studies of equol producers versus non-producers have suggested that equol production may be important in determining benefits of soy consumption in terms of bone health, menopausal symptoms, and breast cancer, although the data are not consistent.4
Microbes eat what we eat: amino acids, sugar, carbohydrates, fiber, fats, or polyphenols. You will see on the OMX Quick Reference Guide that the microbes that make an organic acid metabolite are listed as well as what they use from the diet to make it — amino acids, for example. This means that the presence of a microbial organic acid is not only a sign that gut bacteria are making it, but also that the dietary precursors are available in the gut to be acted upon by those microbes.
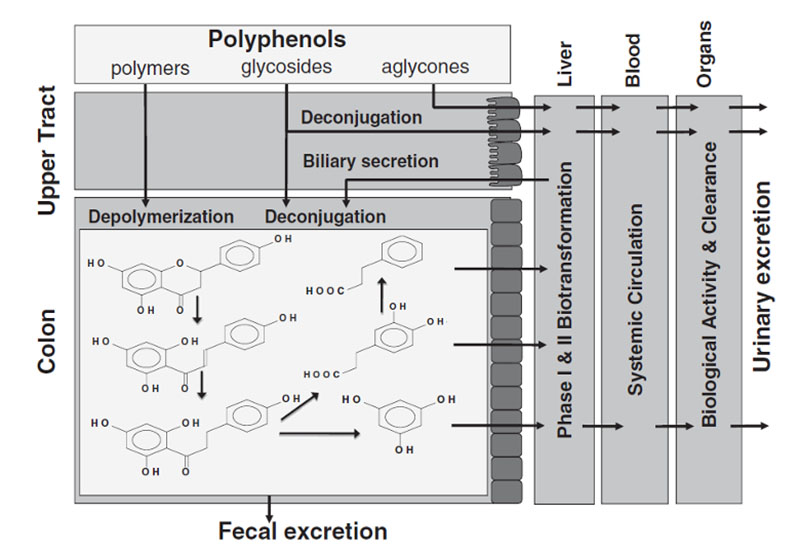
A schematic depiction of how the polyphenol, naringenin, is metabolized by the human-microbial superorganism. Dietary polyphenols and their metabolic byproducts undergo phase I and phase II detoxification, they are absorbed into systemic circulation, they interact with organs and tissues, and they are excreted in urine. Figure used with permission.5
While over 10% of the human metabolome is directly associated with the gut microbial metabolism, specific metabolites are largely uncharacterized. Therefore, methods for the identification and quantification of microbiota-associated metabolites in biological fluids such as urine or plasma are necessary in order to elucidate the molecular basis of host-microbiota interaction.1
Testing and Treatment Considerations
The OMX test is ideal for patients who have a wide range of symptoms affecting not only the gut, but also neurologic, metabolic, detoxification, or oxidative stress systems, as shown here. Use the OMX test in conjunction with the GI-MAP stool test for patients complaining of gut symptoms such as constipation, diarrhea, gas, bloating, reflux, distension, irritable bowel syndrome, or inflammatory bowel disease.
When urine OMX microbial markers are high, along with dysbiosis symptoms, consider treatment with a 5R protocol. High levels of microbial metabolites might be addressed this way:
- Evaluate and optimize the diet — encourage a whole foods diet, low in sugar and refined carbohydrates, balanced in protein, and high in fiber.
- Promote the gut microbiome — give fiber, probiotics, and prebiotics, as tolerated.
- Improve digestion to eliminate microbial overgrowth — support with digestive enzymes, betaine HCl, and good mealtime habits that promote rest, relaxation, and chewing.
- Herbal antimicrobial agents — may be used to lower bacterial and fungal dysbiosis, as needed.
When OMX microbial metabolites are elevated, but the patient has no dysbiosis symptoms, query the patient on their diet. Higher intake of polyphenols or protein could increase levels of the markers. Also consider gentle interventions such as optimizing the diet, supporting healthy gut mucosa, enhancing digestion, and building the beneficial bacteria of the gut microbiome with probiotics, prebiotics, and fiber. Address other abnormalities on the test.
If the OMX bacterial metabolites are all normal or low this is generally considered a positive finding. It suggests no bacterial or fungal overgrowth. However, look for signs that your patient may have poor dietary intake of protein and plant-based polyphenols. Amino acids and polyphenols are food sources for the microbes that might be in short supply if bacterial metabolites are low.
Gut Bacteria Metabolites on the OMX Organic Metabolomics Test
| OMX Urine Marker | Microbial Producers | Dietary Sources | If Low or Normal | If High |
|---|---|---|---|---|
| 4-Hydroxyphenylacetic acid |
|
Tyrosine, tyramine, and polyphenols | Generally considered a positive finding. Suggests low bacterial or fungal overgrowth. A pattern of lows, however, may point to poor intake of polyphenols or amino acids. | Evaluate intake of dietary sources. If gut dysbiosis symptoms are present, consider a 5R protocol including improving the diet, promoting beneficial gut bacteria, enhancing digestion, and antimicrobial agents if needed. Compare with findings from GI-MAP. |
| Indoleacetic acid |
|
Tryptophan | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| Phenylacetic acid
Found in the "Phenylalanine Metabolism" section of the OMX report |
|
Phenylalanine, polyphenols | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| 3,4-Dihydroxyhydrocinnamic acid |
|
Polyphenols – flavonoids | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| 3,5-Dihydroxybenzoic acid | Mixture of unspecified gut microbiota | Polyphenols, hydroxybenzoates | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| 4-Hydroxybenzoic acid |
|
Polyphenols, anthocyanidins | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| Benzoic acid and Hippuric acid |
|
Polyphenols | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| Homovanillic acid
Found in the "Phenylalanine Metabolism" section of the report |
|
Polyphenols from apples, pears, or olives. Quercetin. | Same as above. See: 4-Hydroxyphenylacetic acid | Same as above. See: 4-Hydroxyphenylacetic acid |
| Equol |
|
Soy isoflavones | Indicates poor intake of soy isoflavones and/or the person is not an equol-producer. | Indicates patient is an equol-producer and eats soy isoflavones in the diet. High levels may have benefits for bones, cardiovascular, and endocrine systems. |
| Arabinitol | D-arabinitol is produced by:16
|
Arabinose, glucose, or glycerol. | Generally considered a positive finding. Suggests low bacterial or fungal overgrowth. | If consistent with fungal overgrowth symptoms, consider a 5R protocol with dietary restriction of sugars and starches, promoting beneficial gut bacteria and fungi, enhancing digestion, and antifungal agents if needed. |
| D-Lactate and L-Lactate
Found in the "Glycolysis" section of the OMX report |
|
Carbohydrates | Same as above. See: Arabinitol | If consistent with gut dysbiosis symptoms, consider a 5R protocol including improving the diet (especially limiting carbs), promoting beneficial gut bacteria, enhancing digestion, and antimicrobial agents if needed. Compare with findings from GI-MAP. |
Gut Bacteria Byproducts of Amino Acid Fermentation
4-Hydroxyphenylacetic acid is a microbial byproduct of tyrosine, tyramine, and polyphenols such as those found in wine or green tea. 4-hydroxyphenylacetic acid was the most abundant urinary polyphenol in a study of 475 people and its presence was attributed entirely to microbiota.11 Bacteria believed to produce 4-hydroxyphenylacetic acid are:20
- Arthrobacter species6
- Bacillus species6
- Halomonas species6
- Clostridium species
- Klebsiella species
- Proteus species
- Pseudomonas species
In one study of children, higher 4-hydroxyphenylacetic acid was associated with Giardia lamblia, ileal resection, and small intestine bacterial overgrowth. Researchers noted it as a useful small intestinal bacterial overgrowth (SIBO) screening tool.21 Levels of this urinary polyphenol were 1.4 times higher in men than women.11
The most abundant urinary polyphenols detected in our [human] study were phenolic acids formed by the microbiota: 4- and 3-hydroxyphenylacetic acids, 3,4-dihydroxyphenylacetic acid, protocatechuic acid (and their O-methylated metabolites: homovanillic acid and vanillic acid, respectively), 4-hydroxybenzoic acid, 3,5- and 3,4-dihydroxyphenylpropionic acids, and, 3,5-dihydroxybenzoic acid, with median excretion levels ranging from 3.4 to 157 μmol/24 h.11
Indoleacetic acid (or indole-3-acetic acid) is one of the predominant tryptophan microbial metabolites in the intestine. Indoles are organic chemical structures found in nature and made by gut microbiota. Indoleacetic acid is recognized as a marker of microbial activity.1 Indoleacetic acid levels were high in patients with autism spectrum disorder.22
These gut bacteria are believed to produce indoleacetic acid:
- Bifidobacterium1
- Bacteroides1
- Bacillus subtillis1
- Pseudomonas aeruginosa1
- Clostridium species7
- Clostridium sporogenes8
- Lactobacillus species7
Microbes in the gut are instrumental in tryptophan metabolism. They can impact serotonin, kynurenine, and indole metabolites such as indoleacetic acid, with subsequent effects on brain function. Authors suggest this could be one mechanism underlying the gut-brain axis. They suggest treating the gut microbiota as a strategy to correct brain function and tryptophan metabolism.7
Phenylacetic acid (found in the "Phenylalanine Metabolism" section of the OMX report)
Phenylacetic acid is a breakdown product of phenylalanine and can increase when phenylalanine hydroxylase is impaired. It can be produced by fermentation of phenylalanine by Bacteroides spp.9 It can also be created from metabolism of polyphenols by gut microbes.4,23 Phenylacetic acid is one of the main phenolic metabolites found at high levels in stool.4 It has been positively associated with Crohn's disease and negatively with F. prausnitzii.24,25 Phenylacetic acid was negatively associated with Clostridium cluster XIVa and positively associated with Lactobacillus spp.26
Microbes that make phenylacetic acid include:4
- Bacteroides spp.9
- Bifidobacterium lactis
- Clostridium barlettii
- Escherichia coli
- Eubacterium hallii
- Lactobacillus gasseri
Gut Bacteria Products of Polyphenol Metabolism
3,4-Dihydroxyhydrocinnamic acid (also known as 3,4-dihydroxyhydrocinnamate or 3,4-dihydroxyphenylpropionate) is a major metabolite of microbiota acting on polyphenols, found in human urine.11 3,4-dihydroxycinnamic acid has antioxidant properties and can inhibit secretion of pro-inflammatory cytokines.
Microbes that produce 3,4-dihydroxyhydrocinnamic acid are:10,11
- Bifidobacterium lactis
- Clostridium orbiscindens
- Enterococcus avium
- Escherichia coli
- Eubacterium ramulus
- Lactobacillus gasseri
3,5-Dihydroxybenzoic acid (3,5-DHBA) is a microbial metabolite of polyphenols and hydroxybenzoates. It is high in human urine as a result of microbial activity on dietary polyphenols. 3,5-Dihydroxybenzoic acid is considered a dietary biomarker of non-white bread and cereal intake.11 Levels of 3,5-DHBA in the stool increase after red wine intake, pointing to bacterial metabolism of red wine polyphenols.27 A mixture of fecal microbiota acted on black tea to produce 3,5-dihydroxybenzoic acid in cell studies.28
4-Hydroxybenzoic acid, or p-hydroxybenzoic acid, is a phenolic derivative of benzoic acid. It is found in all living species, from bacteria to plants to humans. 4-Hydroxybenzoic acid is produced when Clostridium saccharogumia and Eubacterium ramulus act on anthocyanidins such as malvidin.4 In a human study looking at microbial polyphenol metabolites, 4-hydroxybenzoic acid was one of the most abundant polyphenols found in urine.11 It correlated with level of education and was lower in subjects with no or only primary education.
4-Hydroxybenzoic acid increased on a low FODMAP diet, and was found to be positively associated with Firmicutes, Verrucomicrobia, and Akkermansia muciniphilia, and negatively with Actinobacteria.29 Coenzyme Q10 is synthesized in multiple steps from the precursor 4-hydroxybenzoic acid.
Microbes that make 4-hydroxybenzoic acid include:
- Clostridium saccarogumia
- Eubacterium ramulus
- Akkermansia muciniphila
Deployment of in vitro gut models, humanized mouse models, and human intervention trials, in combination with deployment of metabolomics and microbiomics, is a prerequisite for unraveling the role of colonic microbiota in the bioconversion of polyphenols. –From Metabolic Fate of Polyphenols in the Human Superorganism by Duynhoven and colleagues5
Benzoic acid and hippuric acid — these two urine metabolites travel together. They are well-known byproducts of bacterial metabolism on dietary polyphenols. Benzoate and hippurate as microbial byproducts have been detected in cell studies, animal studies, and human studies. After treatment with antimicrobials, the levels of these byproducts decrease in urine, attesting to the role of the microbiota in their production.30,31
Examples of polyphenol-containing foods that can produce urine benzoic acid and hippuric acid include: fruit juice, tea, wine, cinnamon, cloves, tomatoes, berries, plums, apples, and cranberries. For example, an increased intake of cranberry polyphenols resulted in an increase in benzoic acid. The increase was individual and likely partially dependent on the gut microbiome.32 Benzoic acid can also be found in processed foods, especially because it is an antimicrobial preservative.
The human body converts benzoic acid to hippuric acid for excretion. Phenolic compounds are converted to benzoic acid, conjugated with glycine and excreted as hippuric acid.33 Glycine and pantothenic acid are needed for this reaction.
Hippuric acid is known as a gut-derived metabolite commonly associated with a "healthy phenotype." It is a key differentiator between the urine metabolic profiles of lean versus obese or diabetic people. It is decreased in diabetes and obesity and increases after bariatric surgery. Increased levels of Collinsella correlated with higher urinary hippurate.12 Collinsella was found to correlate with urinary hippurate in an animal model.
Microbes that produce benzoic acid and/or hippuric acid:
- Escherichia coli4
- Bifidobacterium lactis4
- Lactobacillus gasseri4
- Collinsella12
Benzoic acid and hippuric acid are general microbiota markers. The species that make them are not widely known or published.
Homovanillic acid (HVA) is commonly known as the human breakdown product of dopamine and is found in the "Phenylalanine Metabolism" and "Stress & Mood" sections on the OMX report. However, homovanillic acid is also a significant microbial urine metabolite.34
Urine homovanillic acid was one of the highest microbial metabolites found in a human study of dietary polyphenols intake. Levels of HVA were attributed to microbiota and endogenous synthesis. Levels of HVA were higher in men, in subjects who ate more calories, and in patients who had eaten polyphenols from apples, pears, or olives around the time of the test.11 Quercetin intake can cause very high elevations of urine HVA.35
These organisms may produce HVA:13
- Klebsiella spp.
- Serratia spp.
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
Equol is a product of bacterial metabolism on isoflavones (a type of polyphenol) from soy. Reductase enzymes secreted by the gut microbiota convert daidzein into equol. Daidzein is a phytoestrogen found in soybeans and other legumes with an almost identical chemical composition to mammalian estrogens. Only 20–30% of people in Western countries are equol producers, whereas 40–60% of people in Asian countries are equol producers. All animals tested so far can produce equol.15 Consumption of soy isoflavones alone does not mean someone is an equol producer. It depends on their gut microbiome.14
Aside from its role as a marker of microbial activity in the gut, equol has beneficial health impacts. Equol can modify blood and urine estradiol levels. It is associated with a reduced risk of developing female hormone-related diseases such as breast cancer, hot flashes, and bone loss.4 It has been associated with decreased risks of prostate and colon cancers and cardiovascular diseases.14
These species can produce equol:14,15
- Adlercreutzia equolifaciens
- Asaccharobacter celatus
- Bacteroides ovatus
- Bifidobacterium breve
- Bifidobacterium longum
- Catenibacterium spp.
- Clostridium spp.
- Eggerthella spp.
- Finegoldia magna
- Lactobacillus mucosae
- Lactobacillus paracasei
- Lactobacillus sakei/graminis
- Lactococcus garvieae
- Pediococcus pentosaceus
- Slackia equolifaciens
- Slackia isoflavoniconvertens
- Slackia spp.
- Streptococcus intermedia
Fungal Metabolism Markers
Arabinitol can be found in two forms, as L-arabinitol or D-arabinitol, in human serum and urine. There are some native yeast strains, such as Candida, Debaryomyces, Pichia, and Zygosaccharomyces possessing the ability to biotransform arabinose, glucose, or glycerol to arabinitol.36,37 Elevations of arabinitol should be evaluated for intestinal fungal overgrowth. A ratio of D-arabinitol to L-arabinitol was shown to be elevated with invasive candidiasis in children and in infants infected with C. albicans. Serum D-arabinitol to creatinine ratio correlated with antifungal treatment response in 10 out of 10 adults with cancer.38 Some studies show that serum D-arabinitol can be detected earlier than Candida growth in blood cultures and may therefore be useful for monitoring clinical response to treatment.16
-
D-arabinitol is produced by:16
- Candida albicans
- Candida parapsilosis
- Candida tropicalis
-
Arabinitol can be produced by:
- Debaryomyces
- Pichia
- Zygosaccharomyces
Other Microbial Byproducts Found on the OMX Report
Lactic acid producing bacteria (found in the "Glycolysis" section of the OMX test) D-Lactate and L-Lactate — Lactic acid is made by a wide range of bacteria, yeast, and filamentous fungi.18 Lactic-acid-producing bacteria can make L(+)-lactic acid, D(-)-lactic acid, the racemate DL-lactate, or a combination of these.17 L-lactic acid is called lactic acid on the OMX report. When D-lactic acid elevates in urine, it can cause a severe type of metabolic acidosis known as D-lactic acidosis. It can present with neurological symptoms such as confusion, memory loss, or poor cognition. It has been observed in animals and humans.19
High urinary D-lactic acid usually occurs due to gastrointestinal dysfunction. It has primarily been reported in patients with short bowel syndrome who have had removal of a section of the small bowel. Changes to the small bowel set the stage for difficulty metabolizing carbohydrates, which can lead to excessive bacterial fermentation in the colon, producing D-lactate. In rare situations, the production of D-lactate can exceed a person's ability to metabolize D-lactate, leading to high circulating levels of D-lactate which can be absorbed in the brain and cause encephalopathy.19 Both urine L-lactic acid and D-lactic acid were elevated in those with irritable bowel disease.39 In one case report of short bowel syndrome, probiotic therapy was able to suppress D-lactate producing bacteria.40 Probiotic therapy with D-lactate-producing microbes are not believed to pose a concern for anyone except those with short bowel syndrome17 or malabsorption syndromes.41
Other contributors to urine D-lactate are the human methylglyoxal (MGO) pathway and/or ingestion of preformed D-lactate through fermented foods. MGO is a precursor of glycation of proteins and DNA which is converted into D-lactate when it is detoxified.
These gut bacteria can produce D-lactate, L-lactate, or a mixture:
-
Aerococcus (L-lactate)17
-
Bacillus coagulans (L-lactate)18
-
Carnobacterium (L-lactate)17
-
Enterococcus (L-lactate)17,19
-
Lactobacillus species D-lactate (L-lactate and DL-lactate)17
- Probiotic species that can produce D-lactate include: L. acidophilus, L. gasseri, L. delbrueckii subsp. bulgaricus, L. fermentum, L. lactis, L. brevis, L. helveticus, L. plantarum and L. reuteri.
-
Lactococcus species (L-lactate)17
-
Leuconostoc species (D-lactate)17
-
Oenococcus species (D-lactate)17
-
Pediococcus species (L-lactate and DL-lactate)17
-
Streptococcus (L-lactate)17,19
-
Weissella species (D-lactate and DL-lactate)17
Comprehensive Gut Microbiome Testing
When you combine the GI-MAP stool test and the urine OMX test, a more complete picture of the gut microbiome emerges. It couples direct measurement of gut bacteria, fungi, and parasites in stool (GI-MAP) with the metabolomic fingerprint of the gut microbiota (OMX).
Together, these results can give information about gut bacteria imbalance (dysbiosis), fungal imbalance, if someone is an equol-producer or not, and the dietary products that are being acted upon by the microbiome. Use the table to determine what specific microbes may be involved in elevated urinary organic acids.
As metabolomics research advances, the role of microbes, their byproducts, and how they interact with the human "superorganism" will become clearer. Diagnostics Solutions contributes to a better understanding of the microbial component of the human metabolome with the OMX Organic Metabolomics test.

Author Cass Nelson-Dooley, MS
Cass Nelson-Dooley, MS, studied medicinal plants in the rain forests of Panama, in 2003 as a Fulbright Scholar, and then launched a career in science and natural medicine. She researched the pharmacology of medicinal plants at the University of Georgia and AptoTec, Inc. She has over 15 years of experience teaching doctors about integrative and functional laboratory results.
At Diagnostic Solutions Laboratory, she analyzes GI-MAP stool tests and develops educational tools. Ms. Nelson-Dooley owns Health First Consulting, LLC, a medical writing, patient education, and consulting firm that serves the integrative and functional medicine industry. Ms. Nelson-Dooley is the author of Heal Your Oral Microbiome and has published case studies, book chapters, and journal articles about natural medicine, nutrition, and laboratory testing.
The opinions expressed in this presentation are the author's own. Information is provided for informational purposes only and is not meant to be a substitute for personal advice provided by a doctor or other qualified health care professional. Patients should not use the information contained herein for diagnosing a health or fitness problem or disease. Patients should always consult with a doctor or other health care professional for medical advice or information about diagnosis and treatment.
REFERENCES
- Pavlova T, Vidova V, Bienertova-Vasku J, et al. Urinary intermediates of tryptophan as indicators of the gut microbial metabolism. Anal Chim Acta. 2017;987:72-80.
- Clifford MN. Diet-derived phenols in plasma and tissues and their implications for health. Planta medica. 2004;70(12):1103-1114.
- Armand L, Andriamihaja M, Gellenoncourt S, Bitane V, Lan A, Blachier F. In vitro impact of amino acid-derived bacterial metabolites on colonocyte mitochondrial activity, oxidative stress response and DNA integrity. Biochimica et biophysica acta General subjects. 2019;1863(8):1292-1301.
- Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. European journal of nutrition. 2018;57(1):1-24.
- van Duynhoven J, Vaughan EE, Jacobs DM, et al. Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4531-4538.
- Liebgott PP, Amouric A, Comte A, Tholozan JL, Lorquin J. Hydroxytyrosol from tyrosol using hydroxyphenylacetic acid-induced bacterial cultures and evidence of the role of 4-HPA 3-hydroxylase. Res Microbiol. 2009;160(10):757-766.
- Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr. 2020;11(3):709-723.
- Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716-724.
- He LH, Yao DH, Wang LY, Zhang L, Bai XL. Gut Microbiome-Mediated Alteration of Immunity, Inflammation, and Metabolism Involved in the Regulation of Non-alcoholic Fatty Liver Disease. Front Microbiol. 2021;12:761836.
- Rowland I, Gibson, G., Heinken, et. al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 2018;57:1-24.
- Zamora-Ros R, Achaintre D, Rothwell JA, et al. Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Scientific reports. 2016;6:26905.
- Dewulf EM, Cani PD, Claus SP, et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62(8):1112-1121.
- Chernevskaya E, Beloborodova N, Klimenko N, et al. Serum and fecal profiles of aromatic microbial metabolites reflect gut microbiota disruption in critically ill patients: a prospective observational pilot study. Critical care. 2020;24(1):312.
- Iino C, Shimoyama T, Iino K, et al. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients. 2019;11(2).
- Mayo B, Vazquez L, Florez AB. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients. 2019;11(9).
- Vitetta L, Coulson S, Thomsen M, Nguyen T, Hall S. Probiotics, D-Lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017;8(4):311-322.
- Juturu V, Wu JC. Microbial production of lactic acid: the latest development. Critical reviews in biotechnology. 2016;36(6):967-977.
- Wallis A, Ball M, McKechnie S, Butt H, Lewis DP, Bruck D. Examining clinical similarities between myalgic encephalomyelitis/chronic fatigue syndrome and D-lactic acidosis: a systematic review. J Transl Med. 2017;15(1):129.
- Base FD. 4-Hydroxyphenylacetic acid (FDB010534). 2020; foodb.ca
- Chalmers RA, Valman HB, Liberman MM. Measurement of 4-hydroxyphenylacetic aciduria as a screening test for small-bowel disease. Clin Chem. 1979;25(10):1791-1794.
- Gevi F, Zolla L, Gabriele S, Persico AM. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol Autism. 2016;7:47.
- Tomas-Barberan F, García-Villalba R, Quartieri A, et al. In vitro transformation of chlorogenic acid by human gut microbiota. Molecular nutrition & food research. 2014;58(5):1122-1131.
- Goodwin BL, Ruthven CR, Sandler M. Gut flora and the origin of some urinary aromatic phenolic compounds. Biochem Pharmacol. 1994;47(12):2294-2297.
- Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91.
- Gutierrez-Diaz I, Fernandez-Navarro T, Salazar N, et al. Could Fecal Phenylacetic and Phenylpropionic Acids Be Used as Indicators of Health Status? Journal of agricultural and food chemistry. 2018;66(40):10438-10446.
- Marhuenda-Munoz M, Laveriano-Santos EP, Tresserra-Rimbau A, Lamuela-Raventos RM, Martinez-Huelamo M, Vallverdu-Queralt A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients. 2019;11(11).
- Gross G, Jacobs DM, Peters S, et al. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. Journal of agricultural and food chemistry. 2010;58(18):10236-10246.
- McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241-1251.
- Adamson RH, Bridges JW, Evans ME, Williams RT. Species differences in the aromatization of quinic acid in vivo and the role of gut bacteria. The Biochemical journal. 1970;116(3):437-443.
- Li C, Lee MJ, Sheng S, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13(3):177-184.
- Feliciano RP, Mills CE, Istas G, Heiss C, Rodriguez-Mateos A. Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability. Nutrients. 2017;9(3).
- Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res. 2013;12(4):1527-1546.
- Bitner BF, Ray JD, Kener KB, et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in beta-cells and skeletal muscle cells. The Journal of nutritional biochemistry. 2018;62:95-107.
- Weldin J, Jack R, Dugaw K, Kapur RP. Quercetin, an over-the-counter supplement, causes neuroblastoma-like elevation of plasma homovanillic acid. Pediatr Dev Pathol. 2003;6(6):547-551.
- Kordowska-Wiater M. Production of arabitol by yeasts: current status and future prospects. Journal of Applied Microbiology. 2015;119(2):303-314.
- Christensson B, Sigmundsdottir G, Larsson L. D-arabinitol--a marker for invasive candidiasis. Med Mycol. 1999;37(6):391-396.
- Long SS, Prober CG, Fischer M, eds. Principles and Practice of Pediatric Infectious Diseases. Elsevier; 2018.
- Scheijen JL, Hanssen NM, van de Waarenburg MP, Jonkers DM, Stehouwer CD, Schalkwijk CG. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp Diabetes Res. 2012;2012:234812.
- Yilmaz B, Schibli S, Macpherson AJ, Sokollik C. D-lactic Acidosis: Successful Suppression of D-lactate-Producing Lactobacillus by Probiotics. Pediatrics. 2018;142(3).
- Fabian E, Kramer L, Siebert F, et al. D-lactic acidosis – case report and review of the literature. Z Gastroenterol. 2017;55(1):75-82.